Application of Soft XAFS Technology to Chemical State Analysis of Battery Material (SiO)
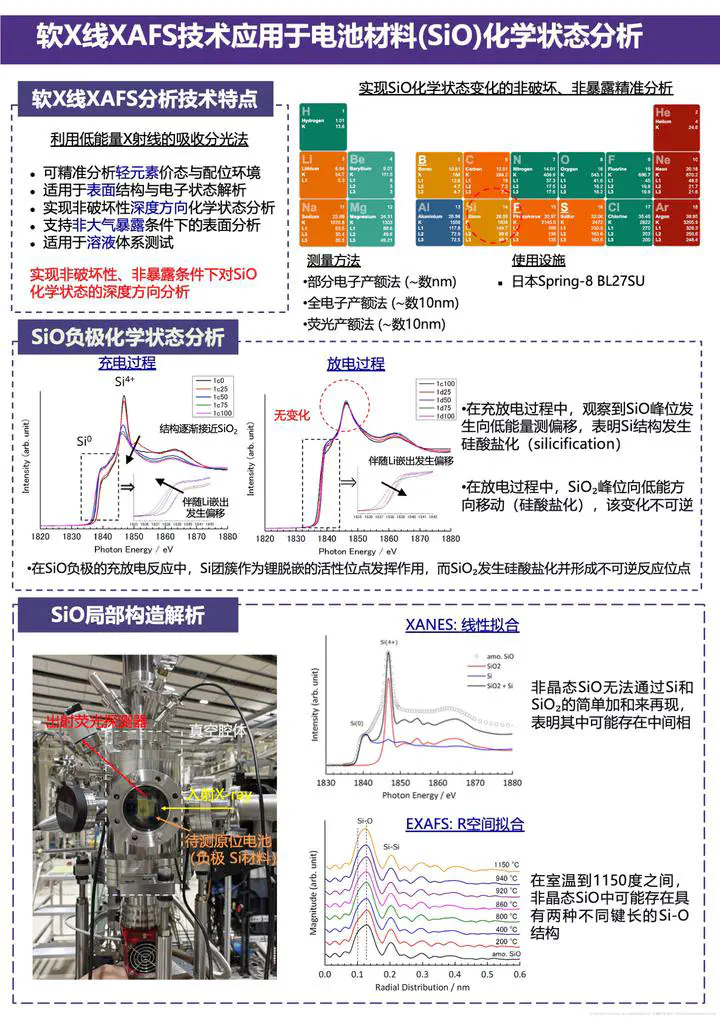 Image credit: Unsplash
Image credit: UnsplashAbstract
By utilizing low-energy X-rays, soft X-ray X-ray absorption fine structure (XAFS) technology was employed at beamline BLSU27 to conduct chemical state analysis of battery material SiO under in situ operando conditions. Soft XAFS enables precise analysis of the valence states and coordination environments of light elements, making it suitable for investigating surface structures, electronic states, and non-destructive depth‑resolved chemical state analysis, while also supporting measurements under non‑atmospheric exposure conditions and in solution systems. Through depth‑resolved chemical state analysis of SiO anode material during charge‑discharge processes, the study revealed that the structure of SiO gradually approaches SiO₂ during charging, accompanied by a shift associated with lithium extraction; during discharging, corresponding changes occur with lithium insertion. This analysis provides critical insight into the structural evolution and electrochemical behavior of battery materials during cycling.
Soft X-ray absorption spectroscopy (soft XAS) is a type of X-ray absorption fine structure (XAFS) spectroscopy technique that utilizes lower-energy X-rays, typically in the range of 100 eV to 2000 eV, commonly referred to as “soft” X-rays. This energy range corresponds to core-level excitations of light elements (such as carbon, nitrogen, oxygen, fluorine) as well as the L-edges of transition metals (such as lithium, silicon, sulfur, manganese, iron, cobalt, nickel, etc.).
Key Features and Applications:
(1) Sensitivity to light elements: It enables direct probing of the electronic structure and chemical states of light elements (e.g., O, Si, Li) that are crucial for battery performance.
(2) Surface and interface sensitivity: Due to the shallow penetration depth of soft X-rays (ranging from several nanometers to a few hundred nanometers), this technique is particularly suitable for investigating chemical changes at the surface, interface, and near-surface regions of electrode materials. This is essential for understanding phenomena such as the solid electrolyte interphase (SEI) and interfacial side reactions.
(3) Chemical state and local coordination environment: By analyzing the energy position, line shape, and fine structure of the absorption edge, precise determination of the oxidation state (valence) and local coordination environment of elements can be achieved.
(4) Non-destructive analysis: Measurements can be performed without damaging the sample.
(5) In situ/operando conditions: With specially designed sample cells, experiments can be conducted under non-atmospheric exposure conditions (e.g., in vacuum or inert gas atmosphere) or even during charging/discharging processes (operando), allowing real-time observation of dynamic changes during electrochemical reactions.