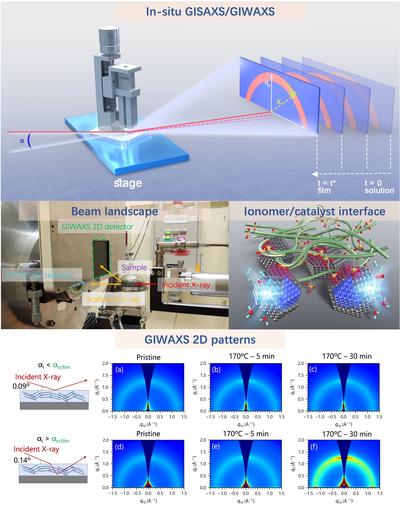
离聚物薄膜
附着于催化剂/离聚物界面(即化学反应发生处)的离聚物纳米薄膜是质子传导和氧传输的媒介。作为聚合物电解质,离聚物薄膜的构象、形貌等微观结构的迁移率从根本上受界面相互作用与空间限域效应的共同影响,共同改变影响着离聚物薄膜的化学与力学性质。
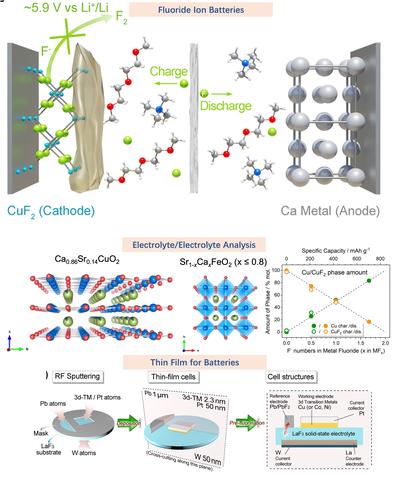
氟离子固态电池
氟离子电池是指一类新兴的固态电池,其采用先进的氟离子化学体系,作为传统锂离子电池的替代方案。与传统的锂离子电池不同,氟离子电池通过固态电解质,使氟离子在金属基负极(如铈、铋)和含氟正极(如氟化铜)之间迁移。这种化学机制因每个离子可转移多个电子,且氟离子本身质量轻,有望实现极高的理论能量密度(可达锂离子电池的5倍)。